Marketing & Advertising
Cindy Eckert’s social media post with a People.com story failed to include any risk information, made misleading representations about benefits and omitted material information about the indication, said the agency, which flagged similar concerns about an Addyi radio ad five years ago.
Two pharmacies, a medical spa and a telehealth company voluntarily removed efficacy, safety and quality claims for compounded GLP-1 products after innovator companies filed challenges with the National Advertising Division.
Health and Human Services Secretary Robert F. Kennedy Jr. said a policy that would remove the tax write-off for pharma advertising expenses would be coming in a few weeks.
Webpages, a healthcare professional pamphlet and a medical conference exhibit booth panel make unsupported comparative superiority claims about generic torsemide products and misrepresent risks, the Office of Prescription Drug Promotion says in its first warning letter of 2025.
A professional slide deck for the drospirenone/estetrol oral contraceptive inappropriately suggests it is safer than other estrogen-containing products and understates risks, Office of Prescription Drug Promotion said in the first “untitled” letter issued since reductions-in-force.
The loss of policy analyst, legal, project manager and social scientist positions has experts wondering if the Trump Administration is eyeing a broader effort to limit DTC advertising. The OPDP layoffs are expected to result in delayed reviews of promotional pieces.
Results from a single-arm study cannot support representations on overall survival, progression-free survival and disease control rate for the cancer drug, the Office of Prescription Drug Promotion said in another “untitled” letter implicating the agency’s CFL guidance.
Sponsors should review longstanding agency concepts on consumer-friendly language and claims limitations, along with Office of Prescription Drug Promotion research and enforcement, when applying the 2018 CFL guidance to direct-to-consumer advertising, Sidley Austin’s Cope says.
A retrospective analysis does not support a claim that multiple myeloma patients are more adherent to Hemady than generic dexamethasone, OPDP said in an "untitled" letter suggesting increased enforcement focus on promotions leveraging the agency’s 2018 CFL guidance.
Sponsors should consider the DTC ad's audio as the major statement about a prescription drug’s side effects and then choose strategically how to display the accompanying text. TV ads are now employing banners and larger text to satisfy the rule’s “dual modality” requirement.
Recent court cases initiated by the Swiss medicines regulator against “unbalanced” media reporting on GLP-1 drugs for weight loss highlights its zero tolerance for any form of misinformation that compromises patient safety.
The US FDA says in a legal memo that its new guidance on scientific information on unapproved uses (SIUU) is “speech-enabling” and argues that challenges should be interpreted as attempts to roll back the approval process.
Patient demand for compounded versions of FDA approved obesity drugs is unlikely to dissipate, even if semaglutide’s “shortage” status is resolved. The issue likely will continue to be a high profile concern for the brand industry.
Lilly’s complaint to the National Advertising Division, a self-regulatory forum for industry, about an Oregon clinic’s claims expands the battlefront against widespread compounding of GLP-1 products for weight loss and diabetes treatment.
Final guidance on communicating scientific information on unapproved uses (SIUU) allows firm-generated presentations to be based on sources other than reprints, but says communications based on nonscientific content are not protected from enforcement.
The compounding industry ties for Martin Makary, President-elect Trump’s candidate to lead the FDA, could mean less compounding enforcement, experts said, but government officials said their enforcement focus will remain nonpartisan.
The loss of institutional knowledge about OPDP’s prior comments on promotional materials can result in an enforcement letter for the new owner of a company or product, experts say.
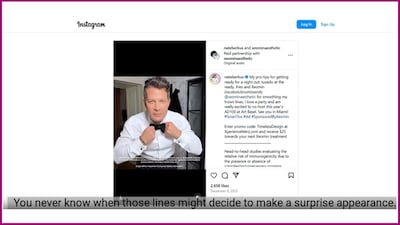
An Office of Prescription Drug Promotion “untitled” letter cites a short-form video by interior designer and TV personality Nate Berkus, continuing an enforcement trend involving drug promotions by social media influencers and celebrities.
Written contracts should be detailed, require compliance with FTC and FDA requirements, and guard against activities that may damage a company’s reputation, experts said.
China has proposed new guidelines designed to head off potential bribery by pharma companies related to the conduct of clinical studies and possible manipulation of outcomes.